“During mastitis, immune cells accelerate glycolysis to support defence, while invading bacteria exploit host metabolism to drive their growth.”
Abstract
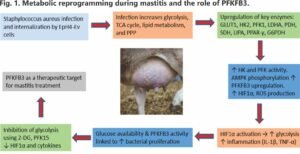
Mastitis, one of the costliest diseases in dairy herds, is increasingly recognized not only as an infectious disorder but also as a metabolic one. During infection, mammary and immune cells undergo profound metabolic reprogramming that fuels both defence and inflammation. Glycolysis is rapidly upregulated, driven by key enzymes such as hexokinase (HK), phosphofructokinase (PFK), and pyruvate kinase (PK), which amplify inflammatory signalling through HIF-1α–mediated cytokine production. At the same time, disruption of the tricarboxylic acid (TCA) cycle leads to the accumulation of succinate—a pro-inflammatory metabolite—and itaconate, which exerts antibacterial and anti-inflammatory effects.
The pentose phosphate pathway (PPP) is also redirected to produce NADPH, sustaining reactive oxygen species (ROS) generation that contributes to pathogen clearance but also causes tissue damage. These interconnected pathways highlight the immune metabolic basis of bovine infectious diseases. Integrating these insights with epitope mapping for diagnostic development, along with the design of potential inhibitors using in silico docking, could further advance both detection and therapeutic strategies. Emerging evidence on these enzymes in mastitis and sepsis highlights their potential as targets for innovative diagnostic and metabolic interventions, offering new opportunities for earlier disease detection, reduced dependence on antibiotics, and more sustainable approaches to udder health management.
Introduction
Mastitis has long been seen as an infectious disease. New research shows it is also a metabolic disorder. During infection, immune and mammary epithelial cells shift from oxidative metabolism to aerobic glycolysis —a rapid but less efficient method of generating energy. This is similar to the Warburg effect described in cancer cells (O’Neill et al., 2016).
 Glycolysis on overdrive: HKII, PFKP, and PKM2
Glycolysis on overdrive: HKII, PFKP, and PKM2
Glycolysis is initiated by hexokinase (HK), which phosphorylates glucose and thereby traps it within the cell. In the context of mastitis, HK functions as the critical gateway enzyme that secures a continuous energy supply. Its expression and activity are closely regulated by signalling pathways such as PI3K/Akt/mTOR, which are activated by insulin and growth factors. This regulation enhances HK transcription and promotes its association with mitochondria, thereby supporting elevated glycolytic flux in proliferating or metabolically active cells. In mastitis, HK activity is increased to secure a continuous supply of metabolic fuel.
Phosphofructokinase (PFK) catalyzes the key commitment step of glycolysis. Increased PFK activity drives glucose deeper into the pathway, sustaining rapid energy production. Under stress conditions such as hypoxia or oxidative stress, upregulation of PFK activity promotes a metabolic shift toward anaerobic glycolysis, ensuring continued ATP production when mitochondrial oxidative phosphorylation is impaired. This metabolic plasticity is particularly important in rapidly dividing cells, including tumour cells and proliferating follicular cells.
A central regulator of this step is PFKFB3, which produces fructose-2,6-bisphosphate, a potent allosteric activator of PFK. In mastitis, PFKFB3 expression in mammary cells is elevated, and its inhibition has been shown to reduce reactive oxygen species generation, suppress HIF-1α signalling, and alleviate Staphylococcus aureus-induced inflammation, underscoring its importance as both a metabolic driver and a potential therapeutic target (Gao et al., 2024), as shown in Fig.1. Targeting glycolytic regulators has therefore emerged as a promising strategy to control this metabolic overactivation.
Pyruvate kinase (PK), produced downstream of PFK, plays a special role. Instead of only working in the cytoplasm, PK can translocate into the nucleus, where it binds to hypoxia-inducible factor 1-alpha (HIF-1α). This complex enhances transcription of pro-inflammatory cytokines, particularly interleukin-1β (IL-1β) (Krawczyk et al., 2010; Rodríguez-Prados et al., 2010; Liu et al., 2022).
The TCA Cycle Stalls and Makes Trouble
Under normal conditions, pyruvate from glycolysis is fed into the TCA cycle, producing a steady supply of energy. But mastitis disrupts this cycle, leading to metabolic bottlenecks that generate inflammatory metabolites.
Succinate accumulates when the cycle is impaired. Succinate acts as a pro-inflammatory metabolite, stabilizing HIF-1α and promoting sustained IL-1β production (Tannahill et al., 2013; Mills & O’Neill, 2014). Itaconate is produced as an alternative pathway product. Itaconate has direct antibacterial effects against pathogens such as Mycobacterium tuberculosis and also reduces inflammation by activating the Nrf2 pathway and restraining type I interferon responses (Michelucci et al., 2013; Mills et al., 2018). Thus, the TCA cycle doesn’t just slow down in mastitis but also actively shapes whether the immune response escalates or resolves.
The Pentose Phosphate Pathway: Fuelling ROS and Inflammation
The pentose phosphate pathway (PPP), which branches off from glycolysis, is also reprogrammed in mastitis. In activated macrophages, PPP activity increases, producing NADPH (Yu et al., 2019). NADPH drives NADPH oxidases, which generate reactive oxygen species (ROS). While ROS are essential for bacterial killing, their overproduction damages mammary tissue and prolongs inflammation (Saito et al., 2021; Ushio-Fukai et al., 2021).
Dysbiosis in the gut has similarly been associated with PPP upregulation, linking gastrointestinal microbial imbalance to mastitis at the immune-metabolic interface (Horst et al., 2021; Kheirandish et al., 2022).
At the same time, the PPP contributes to antioxidant defence. Sedoheptulose kinase (Shpk), a unique enzyme of this pathway, generates sedoheptulose-7-phosphate, which supports NADH production and enhances cellular antioxidant capacity (Haschemi et al., 2012; Nagy & Haschemi, 2015). These findings emphasize the dual nature of the PPP: depending on context, it can exacerbate oxidative stress and inflammation or help restore redox balance and limit tissue injury.
Linking Infection and Metabolism: The TLR4–NF-κB Axis
Pathogen-associated signals are key drivers of the metabolic rewiring observed during mastitis. In particular, Gram-negative bacteria release lipopolysaccharide (LPS), which is recognized by Toll-like receptor 4 (TLR4) on mammary epithelial and immune cells.
LPS binds to LPS-binding protein and CD14, then activates the TLR4–MD2 complex. This recruits MyD88 and activates NF-κB, which enters the nucleus and switches on genes for cytokines such as TNF-α and IL-6 (Chow et al., 1999; Medzhitov, 2009; Akhtar et al., 2020). Beyond its role in cytokine production, NF-κB also stimulates glycolysis, establishing a feed-forward loop in which infection amplifies metabolism and heightened metabolism further sustains inflammation (Sordillo, 2018; Zhao et al., 2022a, 2022b). This tight coupling of immune signalling and metabolic flux highlights why mastitis should be viewed not merely as a localized udder infection but as a systemic metabolic–immune disorder.
From Computational Leads to Farm Solutions
Epitope mapping of HK and PFK isoforms (HK II and PFKP) expressed in mammary epithelial cells offers a promising route to designing multi-epitope vaccines and diagnostic constructs that specifically target key glycolytic enzymes in cattle, forming the basis of affordable diagnostic strips or ELISA kits for early mastitis detection.
Parallel computational docking has highlighted small molecules such as 2-deoxy-D-glucose, Lonidamide, Metformin, and sugar analogs as potential metabolic inhibitors with strong predicted binding affinities. These leads resonate with experimental findings showing that inhibition of PFKFB3, for example with PFK15, can reduce both inflammation and bacterial burden in mastitis (Gao et al., 2024) and in sepsis (Xiao et al., 2023).
Together, these strategies suggest that metabolic enzymes may serve dual purposes: as diagnostic biomarkers for subclinical mastitis and as therapeutic targets for precision interventions. This approach paves the way toward farm-ready solutions that move beyond antibiotics, combining early detection with therapies designed to fine-tune glycolysis and restore immune–metabolic balance.
Conclusion
Mastitis should be understood not only as a bacterial infection but as a metabolic disease. During infection, glycolysis intensifies, with HK, PFK, and PKM fuelling both energy supply and inflammatory signalling. At the same time, the TCA cycle becomes disrupted, releasing metabolites such as succinate, which amplifies inflammation, and itaconate, which tempers it. The pentose phosphate pathway (PPP) accelerates to generate NADPH, feeding both antimicrobial defences and damaging bursts of ROS. Among various regulators, PFKFB3 stands out as a central switch that links infection-driven metabolic rewiring to inflammation.
Emerging tools such as epitope mapping and in silico docking provide a foundation for translating these molecular insights into practice, designing diagnostics that detect disease earlier and metabolic interventions that reduce reliance on antibiotics. Taken together, this integrated view of infection and metabolism points toward a new paradigm in mastitis management: shifting from symptom control to proactive monitoring and metabolic modulation, enabling earlier detection, more precise therapies, and sustainable improvement in udder health.
References are available upon request.
by Sundram Singh and Swati Sangolgi, Master’s (Animal Biochemistry), ICAR-NDRI, Karnal