By Dairy Dimension Policy Desk | Brussels/New Delhi | June 2025
In a critical clarification aimed at regulatory authorities and industry stakeholders, the European Whey Processors Association (EWPA) has formally asserted that standard Whey Protein Isolate (WPI) should not be considered a “novel food” under the EU’s Novel Food Regulation (EU) 2015/2283. The document, issued on June 10, 2025, outlines the technical composition and regulatory standing of WPI, emphasising its long-established use and conventional manufacturing processes.
The announcement is seen as timely and strategic, especially for Indian nutraceutical companies and functional dairy product exporters, as India deepens its engagement in global trade and food regulatory harmonisation.
🧪 What Is WPI and Why Does It Matter
Whey Protein Isolate (WPI) is derived from cow’s milk or whey and is processed to achieve a minimum 90% protein content on a dry matter basis. Through membrane filtration or chromatography, fat, lactose, ash, and moisture are significantly reduced. WPI is widely used in sports nutrition, medical nutrition, and high-protein dairy beverages.
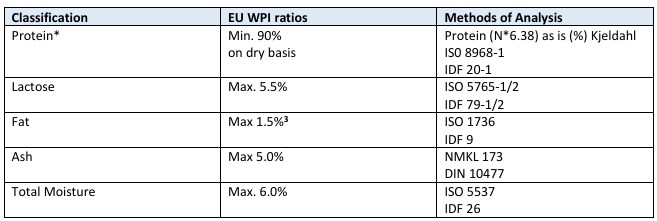
The EWPA document outlines the typical composition:
- Protein (N*6.38): Min. 90%
- Lactose: Max. 5.5%
- Fat: Max. 1.5%
- Ash: Max. 5.0%
- Moisture: Max. 6.0%
These values align with existing food safety benchmarks and have been part of industry-standard manufacturing across Europe for decades.
⚖️ Regulatory Clarity: Not a Novel Food
According to the EWPA, the standard WPI—defined by the composition above and traditional processing methods—does not fall under the “novel food” category. Therefore, it does not require pre-market authorisation under Regulation (EU) 2015/2283.
“WPI has a well-established history of consumption and is produced through non-novel techniques,” the EWPA noted.
This stance, however, does not apply to WPI products with significantly different compositions or novel processing approaches, which would still require individual assessments.
🌍 Why This Matters for India’s Dairy and Health Sector
India is experiencing a rapid increase in demand for high-protein foods and dietary supplements, particularly among urban consumers and fitness enthusiasts. The EU’s clarification can serve as a benchmark for India’s FSSAI, which has been exploring the regulation of protein supplements, including imported whey-based formulations.
- For Indian exporters, especially those looking to ship WPI-based health products to the EU, this clarity lowers regulatory uncertainty.
- For domestic producers, it provides a strong basis for advocating similar clarity under India’s Food Safety and Standards Act, thereby reducing compliance burdens and boosting innovation.
🔄 Potential Trade Impact
As India negotiates agricultural and food trade policies with the EU and the US, standardisation of product categories like WPI will be crucial to:
- Streamline cross-border commerce
- Reduce documentation and approval delays
- Encourage investment in value-added dairy exports and nutraceutical manufacturing
🚀 What’s Next?
- Indian dairy brands exploring sports nutrition SKUs could use this definition to develop EU-compliant product lines.
- FSSAI and Indian trade bodies may consider engaging with EWPA to align domestic guidelines with international standards, ensuring smooth market access and legal certainty.







